SLU-PP-332: Overview
SLU-PP-332 is a low-molecular-weight synthetic small molecule developed as a selective agonist of estrogen-related orphan receptors (ERRs), with primary activity at the ERRα and ERRγ isoforms. Estrogen-related receptors are ligand-activated transcription factors that localize to the nucleus and regulate gene expression programs associated with cellular energy metabolism, mitochondrial biogenesis, and oxidative phosphorylation.
Published Research:
- Metabolic Benefits in Obese Mice: A study published in the Journal of Pharmacology and Experimental Therapeutics demonstrated that SLU-PP-332 administration in diet-induced obese or genetically obese (ob/ob) mice resulted in increased energy expenditure and fatty acid oxidation. These metabolic changes were accompanied by decreased fat mass accumulation, suggesting potential therapeutic applications for metabolic disorders. SLU-PP-332 has been shown to promote weight loss and reduce fat accumulation in obese mice without affecting food intake or requiring increased physical activity. Washington University Profiles
- Enhanced Exercise Endurance: Research reported in ACS Chemical Biology found that SLU-PP-332 increased the proportion of oxidative muscle fibers in mice, leading to enhanced exercise endurance. This effect was attributed to improved mitochondrial function and cellular respiration in skeletal muscle cells. Washington University Profiles
- Potential Treatment for Heart Failure: Studies presented by the American Chemical Society indicated that SLU-PP-332 improved cardiac function in mouse models of heart failure. The compound enhanced mitochondrial ultrastructure and upregulated pathways associated with oxidative phosphorylation and fatty acid metabolism, suggesting its potential as a therapeutic agent for heart failure. American Chemical Society While these findings are promising, it’s important to note that SLU-PP-332 is still in the preclinical stage of development. Further research, including human clinical trials, is necessary to fully understand its efficacy and safety profile.
SLU-PP-332: Biochemical Characteristics and Structure
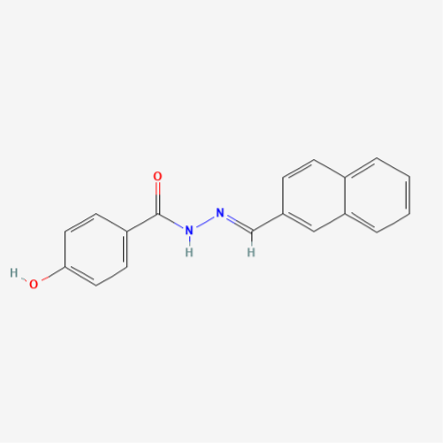
Chemical Formula: C18H14N2O2
Molecular Weight: 290.3 g/mol
PubChem CID: 5338394
CAS No.: 303760-60-3
Synonyms: 4-Hydroxy-N’-(naphthalen-2-ylmethylene) benzohydrazide
What Is SLU-PP-332?
SLU-PP-332 is one of a family of compounds known as estrogen-related receptor agonists (ERRs).
Research indicates that SLU-PP-332 activates the estrogen receptor-related orphan receptors, which are called ERRs for short. ERRs are found with in nucleus of cells and their endogenous (natural) ligand has yet to be unambiguously identified, which is why they are referred to as “orphans.” These receptors come in three types as follows:
- ERRα
- ERRβ
- ERRγ
It is important to note that ERRs, despite their name, are not regulated by estrogen. The name arises from the fact that the gene for ERRα was first isolated due to homology to the gene for the estrogen receptor. That is where their similarities end, however, as evidence to date indicates that estrogen plays no role in the regulation of ERRs.
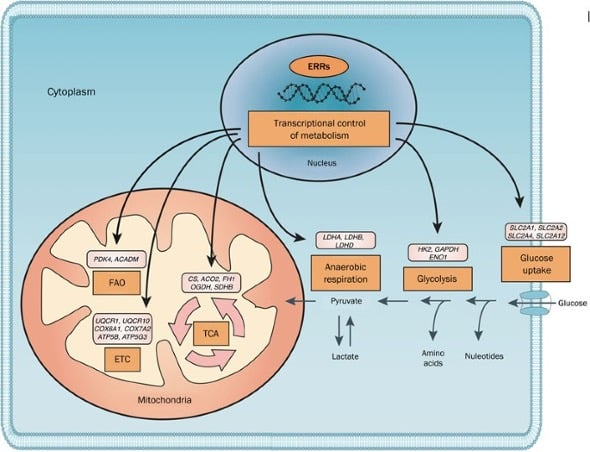
These receptors are known to regulate gene expression patterns with resulting impacts on energy homeostasis, oxidative metabolism, and mitochondrial biogenesis. Stimulation of these receptors can increase energy expenditure and fatty acid oxidation, leading to an increased rate fat loss. They also enhance mitochondrial function, particularly in heart and skeletal muscle cells to improve cardiovascular health as well as exercise tolerance [1].
Source: Nature
One way in which scientists determined that ERRs are important to exercise tolerance is by creating mouse models, called knockouts, that lacked the ERR genes and thus ERRs in skeletal muscle. These mice showed profound intolerance to exercise. ERRα and ERRγ appear to be most important for exercise tolerance, with knockout mice for these two genes showing pale muscles under microscopic examination as well as severe exercise intolerance and decreased oxidative capacity. They also showed an inability to switch to lipid (fat) utilization which is critical for endurance exercise [2].
ERRα regulates genes involved in gluconeogenesis (the production of blood sugar from non-carbohydrate energy stores), fatty acid metabolism, and brown adipose tissue thermogenesis. It also regulates cholesterol, glucose, insulin, and triglyceride levels. It is a necessary receptor for responding to physiological and pathological stresses and has been shown to be a target of the statin class of drugs
ERRγ is much like ERRα. It is an important regulator of mitochondrial activity, plays a vital role in gene transcription, and is a major target of bisphenol A. Interestingly, the binding of bisphenol A (BPA) to the ERRγ receptor may be one reason that BPA has been linked to metabolic syndrome as well as cancer. BPA binding to ERRγ likely interferes with its ability to regulate mitochondrial activity leading directly to glucose dysregulation and, eventually, metabolic syndrome. BPA has long been known to be an endocrine disrupter, showing effects on bone strength and sexual development in mice [3]. Understanding the receptors to which BPA binds not only helps researchers to better counteract the effects of this well-known chemical, it provides deeper insight into mammalian development as well as the pathogenesis of certain disease conditions.
ERRγ is also being investigated for a potential role in the pathogenesis of Parkinson’s disease. It seems that Estrogen-related receptor gamma deficiency may hasten synuclein-mediated toxicity while overexpression can reduce inclusion body load and delay synuclein-mediated toxicity. Thus, ERRγ agonists, like SLU-PP-332 are under investigation as potential treatment in Parkison’s disease [4]. Mitochondrial dysfunction has long been thought to contribute to Parkinson’s disease and thus it makes sense that ERRγ has shown benefit in this condition.
ERRβ is a littler different from the other two ERRs, with its primary function seemingly being to regulate the transition of pluripotent stem cells from one state to another. Thus, ERRβ is likely important in tissue regeneration, growth, and development. It is the least well understood of the ERRs.
SLU-PP-332: Research Applications
SLU-PP-332 is supplied strictly for laboratory research use and has been employed in controlled experimental models to investigate:
- ERRα- and ERRγ-dependent transcriptional regulation
- Mitochondrial biogenesis and ultrastructural remodeling
- Oxidative phosphorylation and fatty acid oxidation pathways
- Cellular energy homeostasis and metabolic gene networks
- Autophagy-associated transcriptional signaling
Estrogen-related receptors function as constitutively active transcription factors that bind ERR response elements within promoter regions of genes governing mitochondrial respiration, lipid metabolism, and oxidative stress regulation. SLU-PP-332 has been shown in preclinical models to enhance ERRα- and ERRγ-mediated transcriptional output.
Downstream effects observed in experimental systems include increased expression of genes associated with electron transport chain assembly, mitochondrial fatty acid uptake, and transcription factor EB (TFEB)-regulated autophagy pathways. These effects position SLU-PP-332 as a mechanistic probe for studying mitochondrial quality control and energy regulation at the transcriptional level.
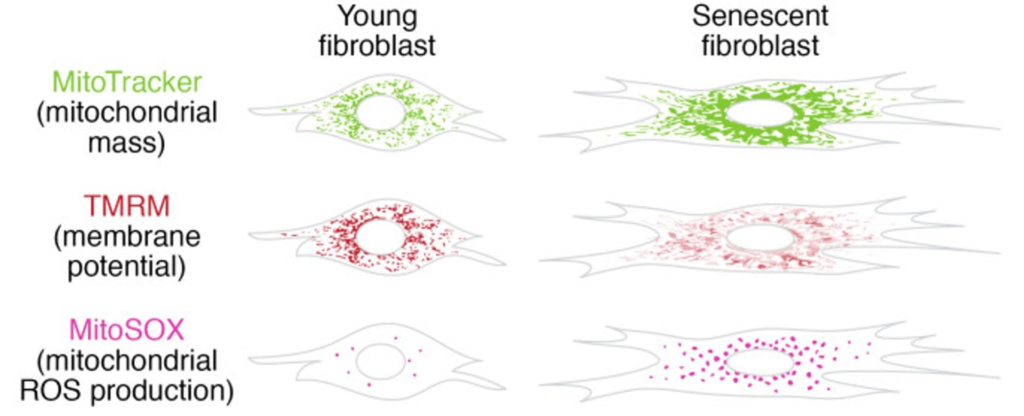
Preclinical investigations utilizing rodent and cellular models have demonstrated that SLU-PP-332 modulates ERR-dependent gene networks governing mitochondrial density, substrate utilization, and oxidative capacity. These studies report alterations in mitochondrial morphology, respiratory efficiency, and lipid oxidation under controlled experimental conditions.
Additional research has examined SLU-PP-332 in models of metabolic stress, cardiac energetic demand, renal mitochondrial aging, and neuronal mitochondrial maintenance, providing insight into conserved ERR-regulated bioenergetic pathways.
Source: PubMed
Are There Other ERR Agonists?
SLU-PP-332 is one of three major ERR agonists currently under investigation. The other two are SLU-PP-1072 and SLU-PP-915. Of the two SLU-PP-1072 is the most similar to 332 in that it primarily interacts with the alpha and gamma ERR variants and has been shown to impact mitochondrial function in skeletal muscle. It has been investigated primarily for its ability to induce apoptosis in prostate cancer cells and is being pursued as a potential treatment for prostate cancer [14].
While similar to it companions, SLU-PP-915 has been primarily investigated for its benefits in heart failure. SLU-PP-915 is structurally distinct from 332, but both have been shown to improve cardiac ejection fraction, decrease fibrosis following cardiac injury, and increase survival rates in mouse models of heart failure. Both ERRα and ERRγ are important regulators of cardiac metabolism [15].
SLU-PP-332: Summary
SLU-PP-332 is an estrogen-related receptor agonist with primary binding proclivities for ERRα and ERRγ. It has more limited binding to ERRβ. Early research has revealed that SLU-PP-332 acts primarily on mitochondria, boosting their capacity to generate energy and reducing oxidative stress as a result. This, in turn, results in increased exercise tolerance and endurance. Additional research has found that SLU-PP-332 can improve heart health, protect the kidneys from the effects of aging, and may help to thwart the pathogenesis of Parkinson’s disease. While research into SLU-PP-332 is just in its infancy, the compound has already shown some remarkable results and has opened up an entirely new area for exploration. SLU-PP-332 is helping scientists to better understand human physiology and will likely lead to the development of a number of therapeutic compounds in the future.
About The Author
The above literature was researched, edited, and organized by Dr. Logan, M.D. Dr. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
Scientific Journal Author
Dr. Hamid Nasri, M.D., is a Clinical Nephropathologist and Nephrologist affiliated with the Department of Natural Sciences at The University of Georgia in Tbilisi, Georgia. He is also a retired Professor of Nephrology as of February 2024.
Dr. Hamid Nasri, M.D., is referenced as one of the leading scientists involved in the research and development of SLU-PP-332. In no way is this doctor/scientist endorsing or advocating the purchase, sale, or use of this product for any reason. There is no affiliation or relationship, implied or otherwise, between Molecular Edge Peptides and this doctor. The purpose of citing the doctor is to acknowledge, recognize, and credit the exhaustive research and development efforts conducted by the scientists studying this peptide. Dr.Hamid Nasri, M.D., is listed in [1] under the referenced citations.
Resources
- H. Nasri, “New hopes on ‘SLU-PP-332’ as an effective agent for weight loss with indirect kidney protection efficacy; a nephrology point of view,” J Ren Endocrinol, vol. 10, no. 1, Art. no. 1, Jan. 2024, doi: 10.34172/jre.2024.25143.
- J.-S. Wattez et al., “Loss of skeletal muscle estrogen-related receptors leads to severe exercise intolerance,” Mol Metab, vol. 68, p. 101670, Jan. 2023, doi: 10.1016/j.molmet.2023.101670.
- F. Xin, L. M. Smith, M. Susiarjo, M. S. Bartolomei, and K. J. Jepsen, “Endocrine-disrupting chemicals, epigenetics, and skeletal system dysfunction: exploration of links using bisphenol A as a model system,” Environ Epigenet, vol. 4, no. 2, p. dvy002, Apr. 2018, doi: 10.1093/eep/dvy002.
- S. N. Fox et al., “Estrogen-related receptor gamma regulates mitochondrial and synaptic genes and modulates vulnerability to synucleinopathy,” npj Parkinsons Dis., vol. 8, no. 1, pp. 1–19, Aug. 2022, doi: 10.1038/s41531-022-00369-w.
- C. Billon et al., “Synthetic ERRα/β/γ Agonist Induces an ERRα-Dependent Acute Aerobic Exercise Response and Enhances Exercise Capacity,” ACS Chem. Biol., vol. 18, no. 4, pp. 756–771, Apr. 2023, doi: 10.1021/acschembio.2c00720.
- J. A. Hawley, M. J. Joyner, and D. J. Green, “Mimicking exercise: what matters most and where to next?,” J Physiol, vol. 599, no. 3, pp. 791–802, Feb. 2021, doi: 10.1113/JP278761.
- “Mimicking exercise with a pill,” American Chemical Society. Accessed: Jan. 23, 2025. [Online]. Available: https://www.acs.org/pressroom/presspacs/2024/march/mimicking-exercise-with-a-pill.html
- “Weight loss: Exercise-mimicking drug may reduce fat, improve insulin.” Accessed: Jan. 23, 2025. [Online]. Available: https://www.medicalnewstoday.com/articles/new-drug-may-help-lose-weight-reduce-fat-by-mimicking-exercise
- P.-M. Badin et al., “Exercise-like effects by Estrogen-related receptor-gamma in muscle do not prevent insulin resistance in db/db mice,” Sci Rep, vol. 6, no. 1, p. 26442, May 2016, doi: 10.1038/srep26442.
- W. Xu et al., “Novel pan-ERR agonists ameliorate heart failure through enhancing cardiac fatty acid metabolism and mitochondrial function,” Circulation, vol. 149, no. 3, pp. 227–250, Jan. 2024, doi: 10.1161/CIRCULATIONAHA.123.066542.
- M. Losby et al., “The Estrogen Receptor-Related Orphan Receptors Regulate Autophagy through TFEB,” Mol Pharmacol, vol. 106, no. 4, pp. 164–172, Sep. 2024, doi: 10.1124/molpharm.124.000889.
- X. X. Wang et al., “Estrogen-Related Receptor Agonism Reverses Mitochondrial Dysfunction and Inflammation in the Aging Kidney,” Am J Pathol, vol. 193, no. 12, pp. 1969–1987, Dec. 2023, doi: 10.1016/j.ajpath.2023.07.008.
- S. Miwa, S. Kashyap, E. Chini, and T. von Zglinicki, “Mitochondrial dysfunction in cell senescence and aging,” J Clin Invest, vol. 132, no. 13, p. e158447, Jul. 2022, doi: 10.1172/JCI158447.
- E. Schoepke et al., “A Selective ERRα/γ Inverse Agonist, SLU-PP-1072, Inhibits the Warburg Effect and Induces Apoptosis in Prostate Cancer Cells,” ACS Chem Biol, vol. 15, no. 9, pp. 2338–2345, Sep. 2020, doi: 10.1021/acschembio.0c00670.
- A. Rodríguez, “Not committed to fail: novel approach improves heart failure outcomes in animal model,” Baylor College of Medicine Blog Network. Accessed: Jan. 23, 2025. [Online]









Reviews
There are no reviews yet.